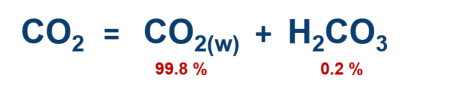Simplified carbonic acid system
If
one
has
to
perform
calculations
in
the
carbonic
acid
system,
one
tries
to
make
the
system clearer and simplifies it.
One
omits
the
specification
of
the
carbonic
acid
itself,
since
it
is
present
only
in
very
small concentrations in the water.
The
reaction
equation
is
presented
as
if
hydrogen
carbonate
is
formed
directly
from
CO
2
and
water.
To
keep
the
calculations
accurate
anyway,
the
two
mass
action
constants of the initial reactions are combined into a new one.
The expanded CO
2
is used here without index.
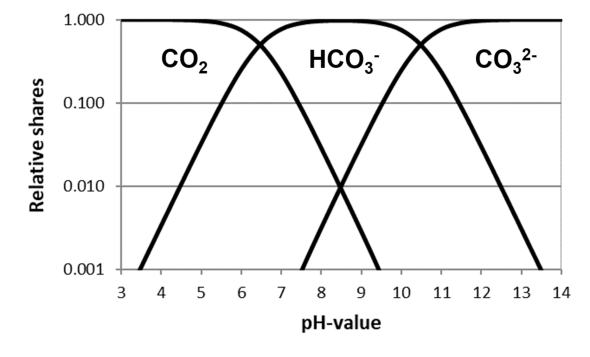
I
In the simplified carbonic acid system, there are then 3 carbonic acid forms:
CO
2
HCO
3
-
CO
3
2-
Carbon dioxide Hydrogen carbonate Carbonate
Also
in
this
simplified
carbonic
acid
system,
the
carbonic
acid
forms
can
be
summarized
in one equation.
The
carbonic
acid
forms
are
present
in
different
proportions
in
the
water.
How
large
the
proportions are depends solely on the pH value (H
+
) of the water.
If
the
pH
is
low,
i.e.
there
are
many
H
+
ions
in
the
water,
the
reaction
equilibrium
is
shifted
completely
to
the
left,
there
is
predominantly
CO
2
in
the
water,
little
hydrogen
carbonate and virtually no carbonate.
If
the
pH
is
high,
i.e.
there
are
few
H
+
ions
in
the
water,
the
reaction
equilibrium
is
shifted
to
the
far
right,
there
is
predominantly
carbonate
in
the
water,
little
hydrogen
carbonate
and virtually no CO
2
.
In
the
medium
pH
range,
hydrogen
carbonate
predominates
and
the
proportions
of
CO
2
and carbonate are low.

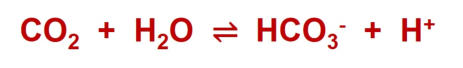
Anker