
Calcite Saturation
Calcite saturation = Lime - carbonic acid equilibrium
Lime - carbonic acid equilibrium
If
the
lime-carbonic
acid
equilibrium
prevails
in
a
water,
then
all
reactions
involved
are
in
reaction equilibrium.
Detailed diagrams on this can be found in the
textbook
.
Lime is neither dissolved nor does it precipitate.
Dissolution and precipitation of lime
Calcite dissolves physically in water up to the solubility limit, which is characterized by the solubility product L. The solubility product is defined by the acivities of the substances a().
If
a(Ca
2+
)∙a(CO
3
2-
) > L
then the water is lime separating
If
a(Ca
2+
)∙a(CO
3
2-
) = L
then the water is in equilibrium
(calcite saturation)
If
a(Ca
2+
)∙a(CO
3
2-
) < L
then the water is lime dissolving
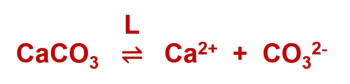
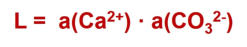
The solubility product for calcite at 10°C is :
L = 3.92∙10
-9
(mol/L)
2
.
Calcite is poorly soluble.
The
purely
physical
solution
of
calcite
in
water
always
leads
only
to
calcite
saturation.
The solubility product can thus not be exceeded.
Calcite
precipitation
always
occurs
when
the
solubility
product
of
calcite
is
exceeded,
for example:
>
A
water
saturated
with
calcite
is
heated.
The
solubility
(L)
of
calcite
decreases
with
increasing temperature, so that the solubility is then exceeded. (e.g. scale formation).
>
CO
2
escapes
from
a
calcite
saturated
water
to
the
air.
This
increases
the
pH
value
of
the
water,
so
that
the
proportion
of
carbonate
in
the
water
increases.
The
solubility
product is thus exceeded and lime can precipitate.
(e.g. formation of swelling lime).
>
In
water
saturated
with
calcite,
the
pH
of
the
water
increases
due
to
other
processes,
e.g.
photosynthesis,
so
that
the
proportion
of
carbonate
in
the
water
increases.
The
solubility product is thus exceeded and lime can precipitate.
(e.g. formation of sea chalk).
All three processes can reinforce each other.
Characterization variables for the behavior of carbonic acid against lime
- Delta pH value ΔpH ΔpH = pH - pH C The delta pH is the difference between the current pH of the water and the pH of calcite saturation, adjusted with calcite. The delta-pH value can be measured. ΔpH < 0 (-) the water is lime dissolving ΔpH > 0 (+) the water is lime separating ΔpH = 0 the water is in equilibrium (calcite saturation) - pH of calcite saturation pH C pH C is the pH value of water at calcite saturation (lime-carbonic acid equilibrium), adjusted with calcite. - Saturation index SI The saturation index is a purely calculated value (cannot be determined by measurement). Like the delta pH value, it indicates the deviation from calcite saturation. The signs are analogous to the delta pH value, the numerical values differ. - Calcite dissolving capacity D(+) The lime dissolving capacity indicates how much lime can be dissolved in a lime dissolving water. The sign for lime dissolving water is (+), in contrast to the delta pH value and saturation index. - Calcite separation capacity D(-) The calcite separation capacity indicates how much lime can be removed from a lime separating water. The sign for lime separating water is (-), in contrast to the delta pH value and saturation index.Causes of lime precipitation in the water cycle
When the solubility product of lime (calcite) is exceeded in water, calcite precipitation
(lime precipitation) occurs.
This can be caused in nature by
- escape of CO
2
into the air
- Photosynthesis (CO
2
withdrawal)
- Temperature increase: The solubility of lime decreases with increasing temperature!
- Increase of the pH value
Anker








