
A
Lime in the water cycle
Spring limestone
Lime precipitation in a spring creek
The groundwater is saturated with lime - in lime-carbonic acid equilibrium. The CO 2 content of the groundwater is always higher than it corresponds to the equilibrium with the atmosphere. So, in the spring CO 2 escapes to the air, the pH value rises and the water in the spring becomes calciferous. This leads to the deposition of lime in the course of the stream. This is particularly evident in the case of a piece of wood from the stream, where half of the lime shell has been removed (figure).
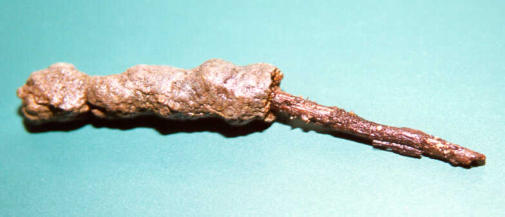
In
the
soil,
the
biological
decomposition
of
organic
matter
produces
further
CO
2
,
which
can dissolve in the percolating water. This increases the dissolution of lime in the soil.
Interim
balance
:
So
we
have
a
water
that
has
been
enriched
with
CO
2
in
equilibrium
with
the
soil
air
and
has
thus
increasingly
dissolved
lime.
The
content
of
CO
2
in
the
water is higher than would be possible in equilibrium with atmospheric air.
Formation of dripstones
If this water is saturated with lime (calcite saturation) and then emerges from the
ground/rock in an underground cave, for example, CO
2
is lost in gaseous form to the
cave air. The process described above is reversed:
Summarized, simplified : Calcite precipitates out of the water and dripstones are formed.
Chemical equation: Increased dissolution of lime due to CO
2
Chemical equation: Precipitation of lime by CO
2
removal
The
same
process
can
occur
with
water
saturated
with
lime
in
a
spring.
CO
2
outgasses
and is lost to the air. Lime precipitates and forms so-called spring limestone.
However,
this
only
happens
in
a
few
wells,
since
the
emerging
waters
are
usually
not
saturated with lime and the outgassing CO
2
then does not lead to lime precipitation.

Lime is contained in rocks of the earth's crust.
Limestone
consists
mainly
of
calcite,
with
a
small
proportion
of
aragonite.
Dolomite
is
a
carbonate
mixture
of
CaCO
3
and
MgCO
3
.
Limestone
and
dolomite
were
formed
by
precipitation
of
lime
and/or
sedimentation
of
calcareous
shells
of
microorganisms
(e.g.
foraminifera) in the primordial oceans.
However, many rocks are free of lime, for example basalt and granite.
Contact with water leads to the dissolution of lime from the rock.
Lime
dissolves
physically
in
the
water
(see
calcite
saturation).
However,
the
solubility
is very low, so that only low concentrations of calcium would be expected in the water.
The presence of carbonic acid in water increases the solubility of lime in water. The natural CO 2 content in rainwater leads to an acidification of the water to pH 5.6. Due to anthropogenic gases (e.g. SO 2 , NO X ), which form acids together with water, the pH of rainwater can be even lower. In the soil, this promotes the dissolution of minerals and lime. Summarized, simplified :
Karstification
Karstification
often
occurs
in
limestone
/
carbonate
rock.
Water
with
dissolved
carbonic
acid
penetrates
fissures
and
crevices
in
the
rock.
As
a
result
of
the
dissolution
of
the
lime,
fissures
become
crevices
in
geological
time
periods
and
some
of
the
crevices
become large cave systems.
An
example
of
such
a
cave
system
is
the
Hölloch
in
the
Mahdtal
(Kleinwalsertal,
Austria)
in
the
area
of
Hoher
Ifen
and
Gottesackerplateau.
The
measured
passage
length
of
this
cave
is
12,900
meters
(http://www.hoelloch.de/index.php).
The
entrance
maw to this cave, the Hölloch, goes 76 meters into the depth.
The
karstified
rock
(Schrattenkalk)
of
the
Hohen
Ifen
and
the
Gottesacker
drains
through
this
cave
system.
The
water
comes
back
to
the
surface
in
three
larger
and
some smaller karst springs.

Figure: Hölloch, 76m deep , access to the karst cave
Stream Shrinkage
Surface water in karst areas can seep into the crevices of the karst, then flow directly underground through these cavities, sometimes forming underground lakes and rivers. If entire streams or rivers disappear underground, this is called stream swallowing (swallow hole), seepage (laminar flow), or sinking (turbulent flow). ( uncertainty about the correct translation ) Well-known is the Danube sinkage near Immendingen (Baden Württemberg, Germany), where at low water the whole river seeps into the ground. At high water, an outflowing residual volume of water remains in the riverbed. The water returns to the surface in the Aach spring (see below).Karst springs
In karst aquifers, the water flows underground at high flow velocities, comparable to surface waters. When the water returns to the surface in karst springs, they have a very high spring discharge. Germany's largest karst springs 1. Aach spring (Radolfzeller Aach, flows into Lake Constance) Average discharge 8,590 liters per second (1,300 to 24,000 L/s) (Most of the water comes from the Danube sinkhole near Immendingen) 2. Blautopf (Blau, flows into the Danube) Average discharge 2,280 liters per second (250 to 32,670 L/s) 3. Rhume spring (Rhume, flows into the Leine) Average flow rate 2,000 liters per second 4. Largest spring area in Germany Pader springs (Pader, flows into the Lippe) All springs together average 5,000 liters per second (3,000 to 9,000 L/s)Estavelle
An Estavelle is both a stream sinkage and a karst spring. At low water levels, it acts as a stream sinkage and absorbs the surface water that flows underground. At high water levels, the groundwater body is filled to such an extent that the estuary overflows and becomes a karst spring. Estavelle during its time as a stream sinkage (Schwarzwasserbach in Kleinwalsertal)See chalk
In lakes, intensive photosynthesis can lead to an increase in the pH value due to CO 2 consumtion. If the lime-carbonic acid equilibrium is exceeded, lime precipitation occurs in the water and lake chalk forms. In Lake Constance, lime deposits are often found on the leaves of aquatic plants (biogenic decalcification). White calcareous sediments in the shore area of Lake Constance are known as "Wysse".
Figure: Danube sinkage near Immendingen (Germany)

Figure: Aach spring in Hegau, Baden Württemberg, Germany
Figure: Blautopf (Blaubeuren) near Ulm, Germany
Figure: Rhumequelle (Rhumspringe, Germany)
Figure: One of the Pader springs (Paderborn, Germany)
Figure: Estavelle in Schwarzwassserbach
(Kleinwalsertal, Austria)
Figure: Wysse in the shore area of Untersee
(Lake Constance, Germany)





Anker
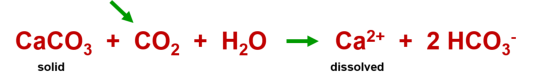
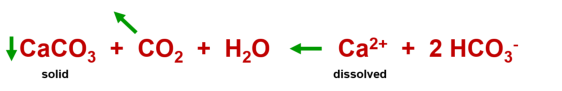
Figure: Dripstone cave
Figure: Spring limestone



























